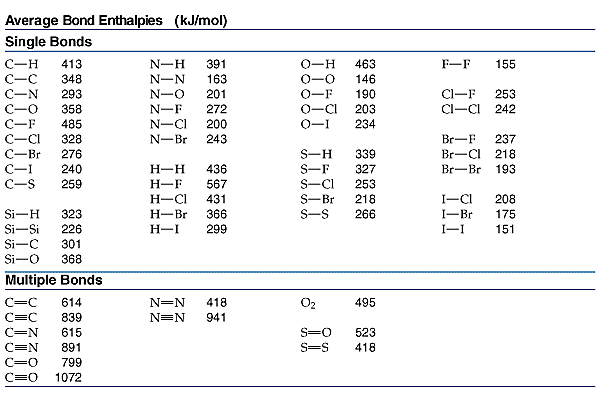
Calculate the number of KJ of heat necessary to raise the temperature of 60.0g of aluminium from.... - YouTube
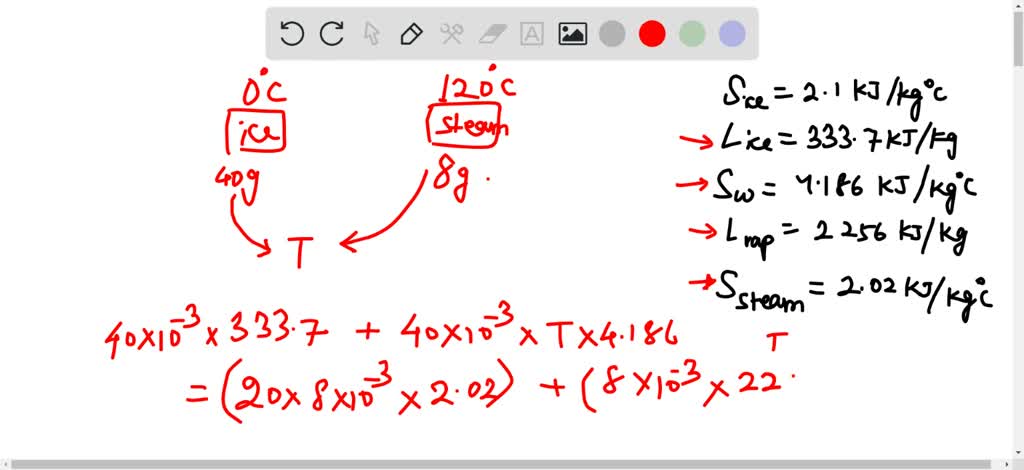
SOLVED: The specific heat of ice is 2.10 kJ/kg C, the heat of fusion for ice at 0 C js 333.7 kJ/kg, the specific heat of water 4.186 kJ/kg C, the heat
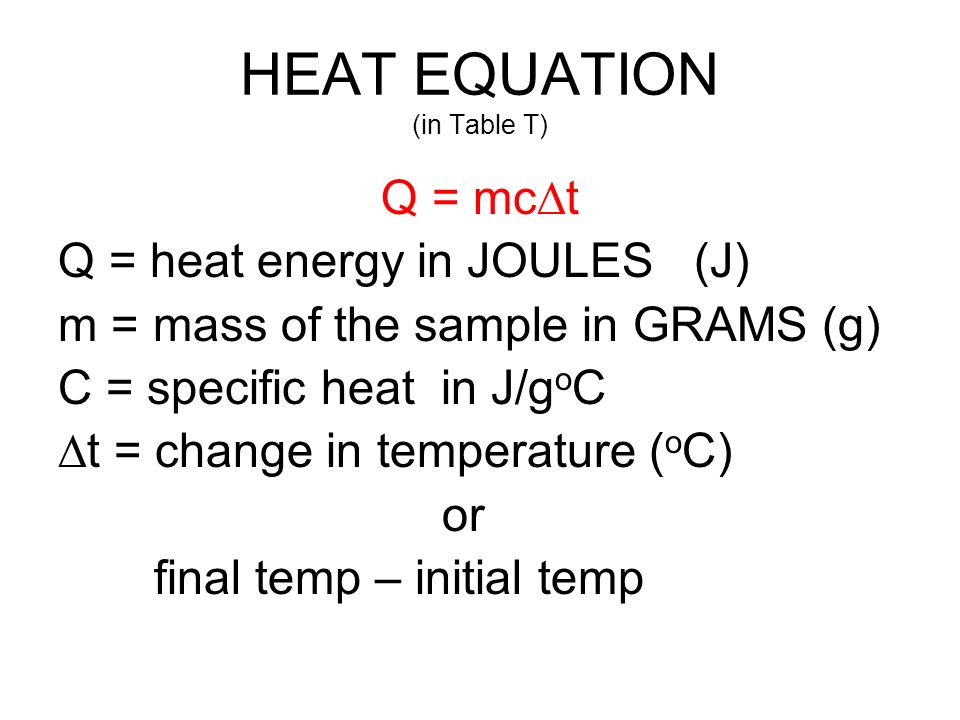
What is the temperature change in 224 g of water upon the absorption of 55 kJ of heat, the specific heat of water is 4.18 J/g °C? | Socratic

A heat pump is used to maintain a house at 25 ? C by extracting heat from the outside air on a day when the outside air temperature is 4 ? C.

USB C Tester,KJ-KayJI 2 in 1 Tester Color Screen IPS Digital Multimeter(2022),Voltage,Cur,Pwr,Resistance,Elec,Temp,Capacity,Tme,Fast Charging,with USB Clip Cable Support PD2.0/PD3.0,QC2.0/QC3.0,BC1.2: Amazon.com: Tools & Home Improvement
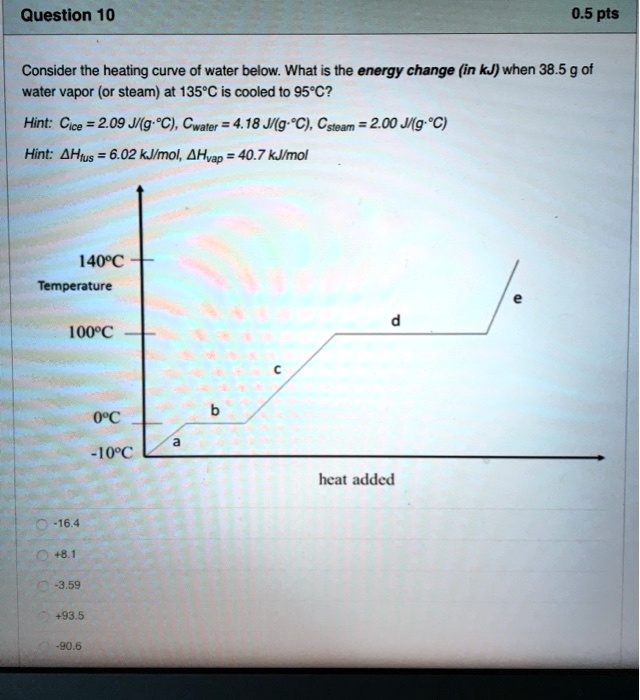
SOLVED: Question 10 0.5 pts Consider the heating curve of water below: What is the energy change (in kJ) when 38.5 9 ot water vapor (or steam) at 135*C is cooled to